Cell migration in cancer
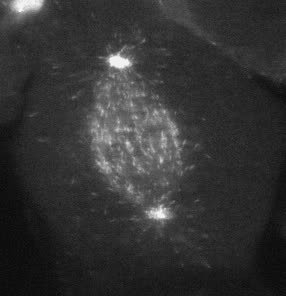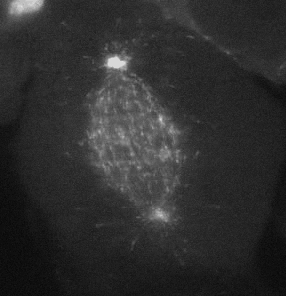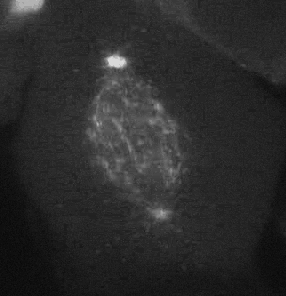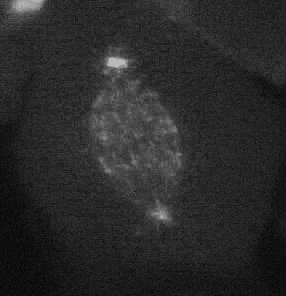
Cell migration is an integrated mechanochemical process that enables a cell to dynamically crawl through its environment. In doing so, immune cells are able to crawl through our tissues on search-and-destroy missions to fight off infections and kill tumors. However, on the darker side, migration also allows cancer cells to spread in our body. Either way, it is important to understand the physical basis of cell migration, so that we can control it for therapeutic purposes.
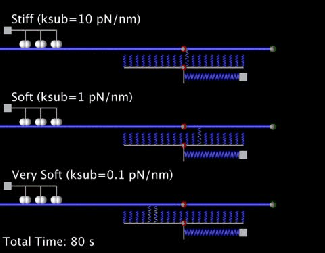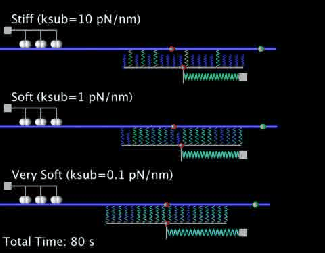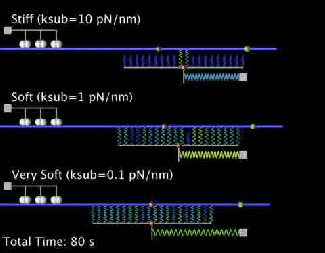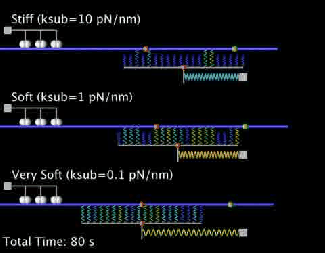
Our current research is focused on the integrated dynamics of F-actin self-assembly, myosin molecular motors, and transmembrane force transmitting molecular clutches. We use computational models to predict the dynamics of cell traction as a function of the chemical and mechanical properties of the environment. We then test these models using live cell microscopy where the cells are transfected with fluorescent proteins fused to key proteins of interest (e.g. actin), and actively collaborate with genome engineers to create mouse tumors and cells that mimic key genetic aspects of the human disease.
A primary focus is on the migration of brain cancer cells (glioblastoma), which is responsible for the deadly spreading of the disease throughout the brain over a period of months, and the T cells that respond to the tumors. By developing computational models tested against in vitro and in vivo migration experiments, we are seeking to identify, in silico, novel therapeutic strategies based on controlling cancer and immune cell migration.
Selected key papers
- Isomursu, A., K.-Y. Park, J. Hou, B. Cheng, G. Shamsan, B. Fuller, J. Kasim, M.M. Mahmoodi, T.J. Lu, G.M. Genin, F. Xu, M. Lin, M. Distefano, J. Ivaska, and D.J. Odde, “Negative durotaxis: cell movement toward softer environments,” Nature Materials (2022).
- Bangasser B.L., Shamsan G.A., Chan C.E., Opoku K.N., Tüzel E., Schlichtmann B.W., Kasim J.A., Fuller B.J., McCullough B.R., Rosenfeld S.S., & Odde D.J.. "Shifting the optimal stiffness for cell migration." Nature Communications, 2017, 8:15313
- Chan C.E. and Odde D.J. “Traction dynamics of filopodia on compliant substrates,” Science, 2008, 322:1687-91.
Modeling and simulation for therapy development
In virtually all areas of technology development, from aircraft to chemical plants to pacemakers, modeling and simulation allow the design of complex systems that are generally safe and effective. By contrast, application of modeling based on physical and chemical principles has not been fully integrated into therapy development in the pharma and biotech industries.
Our approach is to develop a multiscale modeling approach to therapy development to predict, design, and optimize therapeutics interventions. In doing so we aim to move the needle from the current 5% success rate for new therapeutic agents in oncology to at least double, and, hopefully, well beyond.
We recently applied this approach to SARS-CoV-2 viral replication, and predicted the outcomes of clinical trials, including a phase 3, randomized clinical trial that showed a 42% reduction in health care utilization or death relative to placebo control. We aim to apply this approach to cancer, and potentially other diseases as well.
Selected key papers and talks
- Bramante, C.T., J.D. Huling, C.J. Tignanelli, J.B. Buse, D.M. Liebovitz, J.M. Nicklas, K. Cohen, M.A. Puskarich, H.K. Belani, J.L. Proper, L.K. Siegel, N.R. Klatt, D.J. Odde, D.G. Luke, B. Anderson, A.B. Karger, N.E. Ingraham, K.M. Hartman, V. Rao, A.A. Hagen, B. Patel, S.L. Fenno, N. Avula, N.V. Reddy, S.M. Erickson, S. Lindberg, R. Fricton, S. Lee, A. Zaman, H.G. Saveraid, W.J. Tordsen, M.F. Pullen, M. Biros, N.E. Sherwood, J.L. Thompson, D.R. Boulware, and T.A. Murray, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19.New England Journal of Medicine, 2022. 387(7): p. 599-610.
- Castle, B.T., C. Dock, M. Hemmat, S. Kline, C. Tignanelli, R. Rajasingham, D. Masopust, P. Provenzano, R. Langlois, T. Schacker, A. Haase, and D.J. Odde, “Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets,” bioRxiv, p. 2020.05.22.111237 (2020).
- Klank R.L., Rosenfeld S.S., Odde D.J. "A Brownian dynamics tumor progression simulator with application to glioblastoma." Convergence Science Physical Oncology. 2018 Mar;4(1). pii: 015001. doi: 10.1088/2057-1739/aa9e6e.
- How cell phones and video gaming can help cancer patients:
Body-storming: Scientific exploration through human movement
As we construct mathematical models of biological processes, we are unlimited by the model’s complexity. With only a few equations and 5-10 parameters, the complexity of the model quickly surpasses our intuition, so that it is difficult to immediately understand why a model behaves in a certain way. Deconstruction of a mathematical/computational model quickly becomes very time-consuming and rate-limiting.
“Body-storming,” or using bodies and minds to construct and deconstruct models, is a concept we developed in the IAS-funded “The Moving Cell Project” collaborative with Dance Professor Carl Flink and the dance company The Black Label Movement. With dancers following the rules of a simulation, we quickly deconstruct models through observation of the simulation in real time and feedback from the dancers. This helps us quickly test opposing hypotheses and decide which to pursue and disregard based on contradictions with experimental results. Overall, “body-storming” provides visual information on why a model works or fails and streamlines the process of selecting a successful model.
Watch a video of The Moving Cell Project:
Selected key papers and talks
- Flink C. and Odde, D.J. "Science+dance=bodystorming," Trends in Cell Biology, 2012, 22:613-616.
- Walker, S.A., A. Pham, S. Nizzero, M. Kim, B. Riter, J. Bletz, S. Judge, B. Phillips, D. Noble, D. Murray, E. Wetzel, S. Samson, M. McMahon, C. Flink, J. Couch, C. Tomlin, K. Swanson, A.R.A. Anderson, D. Odde, H. Shen, S. Hughes, N. Zahir, H. Enderling, and J. Wolfram, “Education and Outreach in Physical Sciences in Oncology,” Trends in Cancer, 7(1): p. 3-9 (2021).
- David Odde & Black Label Movement: If truth is beauty, can art be science?